 |
 |
Visicol™ (sodium phosphate monobasic monohydrate, USP, and sodium phosphate dibasic anhydrous, USP) is a bowel evacuant used to clean the colon prior to colonoscopy. Visicol™ Tablets are white to off-white modified oval compressed tablets, the upper half bisected with a monogram "I" on both sides and the lower half plain. Each 2.0 gram tablet contains 1.102 grams of sodium phosphate monobasic monohydrate, USP and 0.398 grams of sodium phosphate dibasic anhydrous, USP for a total of 1.5 grams of sodium phosphate. Inert ingredients include microcrystalline cellulose, NF; magnesium stearate, NF; and colloidal silicone dioxide, NF.
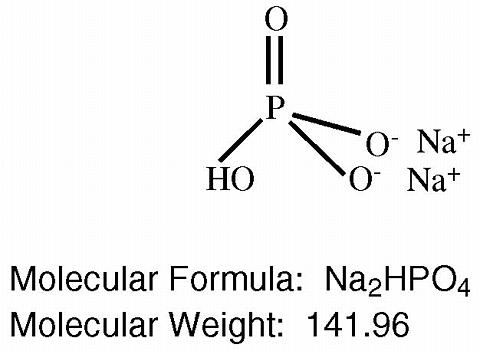
The structural and molecular formulae and molecular weights of the active ingredients are shown below:
Visicol™ Tablets are for oral administration only.
Visicol™ Tablets, taken in two doses of 30 grams approximately twelve hours apart, induce a diarrhea, which rapidly and effectively cleanses the entire colon. Each administration has a purgative effect for approximately 1 to 3 hours. The primary mode of action is thought to be through osmotic action of sodium, causing large amounts of water to be drawn into the bowel, promoting bowel evacuation.
Twenty-three normal healthy volunteer subjects participated in an open-label pharmacokinetic study of Visicol™ Tablets designed to generate concentration-time curves for serum inorganic phosphorus levels after administration of Visicol™ Tablets. Subjects were administered Visicol™ Tablets in accordance with the prescribed labeling for colon cleansing, 30 grams Visicol™ Tablets (20 tablets given as 3 tablets every 15 minutes with clear liquids) at 6 PM in the evening. The 30 gram dose (20 tablets given as 3 tablets every 15 minutes with clear liquids) was repeated the following morning at 6 AM. The serum phosphorus level rose to a maximum mean (± standard deviation) peak of 3.7 ± 1.63 mg/dL above baseline, at a median of 3 hours after the first administration of Visicol™ Tablets. Following the second administration of Visicol™ Tablets the serum phosphorus level rose to a maximum mean (± standard deviation) peak of 4.4 ± 1.86 mg/dL above baseline, at a median of 4 hours.
 |
The serum phosphorus level remained above baseline for a median of 24 hours after the initial dose of Visicol™ Tablets (range 16 to 48 hours), with a maximum mean decrease at 18 hours in serum calcium of 0.3 mg/dL ± 0.3 mg/dL.
Renal insufficiency: The effect of renal dysfunction on Visicol™ Tablets pharmacokinetics has not been studied. Since the ionized, inorganic form of phosphate in the circulating plasma is excreted almost entirely by the kidneys, patients with renal disease may have difficulty excreting a large phosphate load. Thus, Visicol™ Tablets should be used with caution in patients with impaired renal function.
Hepatic insufficiency: Visicol™ Tablets have not been investigated in patients with hepatic failure. The ionized, inorganic form of phosphate in the circulating plasma is excreted almost entirely by the kidneys. Visicol™ is not expected to be metabolized in the liver.
Geriatric: Both AUC and Cmax values of serum phosphate were less than half in subjects >70 years of age compared to subjects <70 years of age, based on the results of a single pharmacokinetic study, which included 6 elderly volunteers. Plasma half-life increased two-fold in subjects >70 years of age compared to subjects <50 years of age (3 subjects and 5 subjects, respectively).
Gender No difference in serum phosphate AUC values were observed in the single pharmacokinetic study conducted in 13 male and 10 female healthy volunteers.
A total of 957 adult patients were enrolled and treated in the controlled clinical trials of Visicol™ Tablets. Males and females were about equally represented. Approximately 87% of the study population was Caucasian. Visicol™ Tablets were found to be comparable in cleansing efficacy to the comparison drug, a commercially available polyethylene glycol-salt (PEG-salt solution) solution (Cherry Flavor NuLYTELY®). Two identical, single (investigator) blind, randomized, multicenter trials were conducted comparing the efficacy and safety of Visicol™ Tablets and the PEG-salt solution comparator as a colon cleansing agent in patients undergoing routine diagnostic colonoscopy. In each study, over 200 patients were randomized to self-administer the Visicol™ Tablets and over 200 were randomized to self-administer the PEG-salt solution comparator. Colonoscopy was generally performed within 5 hours of the second dose. Physicians used a four-point, validated Physician Questionnaire to assess efficacy. The distribution of "excellent", "good", "fair" and "inadequate", as evaluated by the physician performing the colonoscopy, was comparable in both groups. Cleansing efficacy observed in these studies is described in the following table.
|
||||||||||||||||||||||||||||||||||||||||
The efficacy of overall colonic cleansing with the Visicol™ Tablets was comparable to the PEG-salt solution. In addition, the incidence of "Inadequate" colon cleansing ratings due to poor purgative preparation was similar between Visicol™ Tablets and the PEG-salt solution comparator. Also, cleansing efficacy in the ascending colon with Visicol™ Tablets was comparable to the PEG-salt solution.
Visicol™ Tablets are indicated for cleansing of the bowel as a preparation for colonoscopy, in adults 18 years of age or older.
Visicol™ Tablets are not to be used in patients with congestive heart failure, ascites, unstable angina pectoris, gastric retention, ileus or acute obstruction or pseudo-obstruction, severe chronic constipation, bowel perforation, acute colitis, toxic megacolon, or hypomotility syndrome (such as hypothyroidism, scleroderma).
Visicol™ Tablets are contraindicated in patients with a known allergy or hypersensitivity to sodium phosphate salts or any of its ingredients.
Administration of other sodium phosphate-containing products, such as enemas or non-prescription liquid purgatives, has resulted in fatalities due to significant fluid shifts, severe electrolyte abnormalities, and cardiac arrhythmias. These have been observed in patients with renal insufficiency or bowel perforation, and with misuse or overdose of these products.
Use with caution in patients with impaired renal function, pre-existing electrolyte disturbances (such as dehydration or those secondary to the use of diuretics), or people taking drugs that may affect electrolyte levels. Patients with electrolyte abnormalities such as hypernatremia, hyperphosphatemia, hypokalemia, or hypocalcemia should have them corrected before treatment with Visicol™ Tablets.
Patients should be instructed to drink at least 8 ounces of clear liquids with administration of Visicol™ Tablets. Inadequate fluid intake, as with any effective purgative, may lead to excessive fluid loss and hypovolemia.
Undigested or partially digested Visicol™ Tablets may be seen in the watery diarrhea stool or during colonoscopy. In addition, undigested tablets from other medications may be seen.
Patients should be instructed not to administer additional agents, particularly additional sodium phosphate-based purgative or enema products.
Prolongation of the QT interval has been observed in some patients who were dosed with Visicol™ Tablets. QT prolongation with Visicol™ Tablets has been associated with electrolyte imbalances, such as hypokalemia and hypocalcemia. Visicol™ Tablets should be used with caution in patients who are taking medications known to prolong the QT interval, since serious complications may occur. In these studies, prolongation of the QT interval was also observed in some patients treated with PEG-salt solution.
Administration of Visicol™ Tablets may induce colonic mucosal aphthous ulcerations, an endoscopic finding observed with other sodium phosphate cathartic preparations. This colonoscopic finding should be considered in patients with known or suspect inflammatory bowel disease.
Because published data suggest that sodium phosphate absorption may be enhanced in patients experiencing an acute exacerbation of chronic inflammatory bowel disease, Visicol™ Tablets should be used with caution in such patients.
Visicol™ Tablets were not studied in patients within 3 months of an acute myocardial infarction or cardiac surgery, including coronary artery bypass graft surgery, and therefore should be used with caution in such patients.
Medications administered in close proximity to Visicol™ Tablets may not be absorbed from the gastrointestinal tract due to the rapid intestinal peristalsis and watery diarrhea induced by the purgative agent.
Because of the mechanism of action of Visicol™ Tablets, patients should be advised to take only clear liquids by mouth for at least 12 hours prior to starting the purgative regimen.
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Visicol™. Studies to evaluate the effect of Visicol™ on fertility or its mutagenic potential have not been performed.
Category C. Reproduction studies have not been conducted with Visicol™. It is also not known whether Visicol™ can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. Visicol™ Tablets should be given to a pregnant woman only if clearly needed.
Safety and efficacy of Visicol™ Tablets have not been demonstrated for patients less than 18 years of age.
Of the total number of subjects in clinical studies of Visicol™ Tablets, 27.7 percent were over 65, while 8.3 percent were over 75. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. However, greater sensitivity of some older individuals cannot be ruled out. Visicol™ Tablets should be used with caution in elderly patients.
Sodium phosphate is known to be substantially excreted by the kidney, and the risk of adverse reactions with sodium phosphate may be greater in patients with impaired renal function. Elderly patients are more likely to have impaired renal function, and it may be useful to assess renal function.
The most commonly observed adverse reactions (incidence >/=1%, all treated patients) occurring with the use of Visicol™ Tablets are related to the desired purgative effect. Nausea, vomiting, abdominal bloating, abdominal pain, dizziness, and headache occur, and are generally transient and self-limited, requiring no specific treatment. All of these adverse events also occurred in patients who took the PEG-salt solution comparator. Small superficial mucosal ulcerations, typical of those previously reported from the use of liquid preparations of sodium phosphate, and instances of mucosal bleeding have been observed on colonoscopy.
One patient, with no history of heart disease, developed an initial episode of atrial fibrillation after severe vomiting immediately after taking Visicol™ Tablets. Another patient, with known arteriosclerotic heart disease, who took the PEG-salt solution comparator developed atrial fibrillation and also vomited. No patient in the clinical studies developed predefined postural changes in vital signs with concomitant symptoms of lightheadedness or syncope.
Serum electrolyte changes have been observed in patients taking Visicol™ Tablets, primarily affecting calcium, phosphate, and potassium. These changes essentially corrected within 48 to 72 hours after dosing, were not associated with any clinical adverse events and did not require treatment. No patient developed clinically significant hypocalcemia; among patients receiving Visicol™ Tablets 60 grams (n=481) the mean decrease from baseline in calcium level was 0.5 mg/dL. No patient developed a calcium level below 7.1 mg/dL. No clinical adverse events suggestive of hypocalcemia were observed. Mean serum phosphorus levels increased by approximately 3.6 mg/dL transiently after taking Visicol™ Tablets with no apparent clinical significance. A reactive decrease in serum phosphorus 2-3 days after dosing was observed; the mean decrease from baseline at that time was 0.7 mg/dL. The mean decrease in serum potassium after taking Visicol™ Tablets was 0.5 mEq/L.
Under the direction of a physician, Visicol™ Tablets are indicated for cleansing of the bowel when required as a preparation for colonoscopy, in adults 18 years of age or older. Laxatives and purgatives as a group have the potential for abuse by persons with eating disorders who "binge" and "purge".
There have been no reported cases of overdosage with Visicol™ Tablets. Purposeful or accidental ingestion of more than the recommended dosage of Visicol™ Tablets might be expected to lead to severe electrolyte disturbances, including hyperphosphatemia, hypocalcemia, hypernatremia, or hypokalemia, as well as dehydration and hypovolemia, with attendant signs and symptoms of these disturbances. Certain severe electrolyte disturbances may lead to cardiac arrhythmias and death. The patient who has taken an overdosage should be monitored carefully, and treated symptomatically for complications until stable.
The usual adult dosage of Visicol™ Tablets for colon cleansing is 40 tablets taken in the following manner:
The evening before the colonoscopy procedure, 3 Visicol™ Tablets should be taken with at least 8 ounces of clear liquids every 15 minutes (the last dose will be 2 tablets) for a total of 20 tablets. The day of the colonoscopy procedure, (starting 3-5 hours before the procedure) 3 Visicol™ Tablets should be taken with at least 8 ounces of clear liquids every 15 minutes (the last dose will be 2 tablets) for a total of 20 tablets.
Patients are not to repeat this purgative agent within seven days of a previous administration. No additional enema or laxative is required, and patients should be advised NOT to take additional agents, particularly those containing sodium phosphate.
Visicol™ Tablets are supplied in child-resistant bottles containing 40 tablets (60 grams). Each tablet contains 1.102 g sodium phosphate monobasic monohydrate, USP and 0.398 g sodium phosphate dibasic anhydrous, USP for a total of 1.5 g of sodium phosphate. Each bottle contains two silica desiccant packets, which are not to be ingested.
NDC 61607-100-40
Rx only.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]. Discard any unused portion.
Manufactured by:
Pharmaceutical Manufacturing Research Services, Inc.
Horsham, PA 19044
for
InKine Pharmaceutical Company, Inc.
1787 Sentry Parkway West
Building 18, Suite 440
IPI-0001 Revised 11/9/00
©InKine Pharmaceutical Company, Inc. 2000